| ถ่าน | 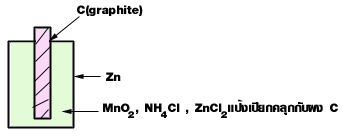
|
| 1. Anode (Oxidation) | Zn | --> | Zn2+ + 2e- |
| 2. Cathode (Reduction) | 2MnO2 + 2NH4 + + 2e- | ---> | Mn2O3 + H2O + 2NH3 |
| Zn + 2MnO2 + 2NH4 + | ---> | Zn2+ + Mn2O3 + H2O + 2NH3 |
เซลล์อัลคาไลน์ มี
| 1. Anode (Oxidation) | Zn + 2OH- | --> | ZnO + H2O + 2e- |
| 2. Cathode (Reduction) | 2MnO2 + H2O + + 2e- | ---> | Mn2O3 + 2OH- |
| Zn + 2MnO2 | ---> | ZnO + Mn2O3 |
เซลล์
| 1. Anode (Oxidation) | Zn + 2OH- | --> | ZnO + H2O + 2e- |
| 2. Cathode (Reduction) | HgO + H2O + + 2e- | ---> | Hg + 2OH- |
| Zn + HgO | ---> | ZnO + Hg |
เซลล์
| 1. Anode (Oxidation) | Zn + 2OH- | --> | ZnO + H2O + 2e- |
| 2. Cathode (Reduction) | Ag2O + H2O + + 2e- | ---> | 2Ag + 2OH- |
| Zn + Ag2O | ---> | ZnO + 2Ag |